Sherman Function on:
[Wikipedia]
[Google]
[Amazon]
 The Sherman function describes the dependence of electron-atom scattering events on the spin of the scattered
The Sherman function describes the dependence of electron-atom scattering events on the spin of the scattered
 When an electron beam is polarized, an unbalance between spin-up, , and spin-down
When an electron beam is polarized, an unbalance between spin-up, , and spin-down
 The Sherman function is a measure of the probability of a spin-up electron to be scattered, at a specific angle , to the right or to the left of the target, due to spin-orbit coupling. It can assume values ranging from -1 (spin-up electron is scattered with 100% probability to the left of the target) to +1 (spin-up electron is scattered with 100% probability to the right of the target). The value of the Sherman function depends on the energy of the incoming electron, evaluated via the parameter . When , spin-up electrons will be scattered with the same probability to the right and to the left of the target.
Then it is possible to write
:
:
Plugging these formulas inside the definition of asymmetry, it is possible to obtain a simple expression for the evaluation of the asymmetry at a specific angle , ''i.e.'':
:.
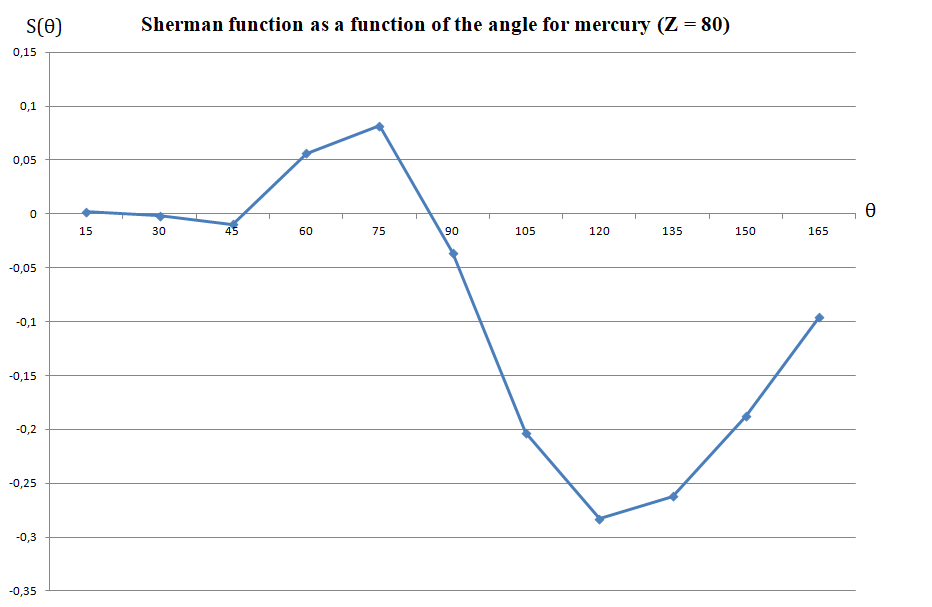
Theoretical calculations are available for different atomic targets and for a specific target, as a function of the angle.
The Sherman function is a measure of the probability of a spin-up electron to be scattered, at a specific angle , to the right or to the left of the target, due to spin-orbit coupling. It can assume values ranging from -1 (spin-up electron is scattered with 100% probability to the left of the target) to +1 (spin-up electron is scattered with 100% probability to the right of the target). The value of the Sherman function depends on the energy of the incoming electron, evaluated via the parameter . When , spin-up electrons will be scattered with the same probability to the right and to the left of the target.
Then it is possible to write
:
:
Plugging these formulas inside the definition of asymmetry, it is possible to obtain a simple expression for the evaluation of the asymmetry at a specific angle , ''i.e.'':
:.
Theoretical calculations are available for different atomic targets and for a specific target, as a function of the angle.
 The Sherman function describes the dependence of electron-atom scattering events on the spin of the scattered
The Sherman function describes the dependence of electron-atom scattering events on the spin of the scattered electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
. It was first evaluated theoretically by the physicist Noah Sherman and it allows the measurement of polarization of an electron beam by Mott scattering
Mott scattering in physics, also referred to as spin-coupling inelastic Coulomb scattering, is the separation of the two spin states of an electron beam by scattering the beam off the Coulomb field of heavy atoms. It is named after Nevill Francis M ...
experiments. A correct evaluation of the Sherman function associated to a particular experimental setup is of vital importance in experiments of spin polarized photoemission spectroscopy
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in th ...
, which is an experimental technique which allows to obtain information about the magnetic behaviour of a sample.
Background
Polarization and spin-orbit coupling
 When an electron beam is polarized, an unbalance between spin-up, , and spin-down
When an electron beam is polarized, an unbalance between spin-up, , and spin-down electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, , exists. The unbalance can be evaluated through the polarization defined as
:.
It is known that, when an electron collides against a nucleus, the scattering event is governed by Coulomb interaction
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is convention ...
. This is the leading term in the Hamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
, but a correction due to spin-orbit coupling can be taken into account and the effect on the Hamiltonian can be evaluated with the perturbation theory
In mathematics and applied mathematics, perturbation theory comprises methods for finding an approximate solution to a problem, by starting from the exact solution of a related, simpler problem. A critical feature of the technique is a middl ...
. Spin orbit interaction can be evaluated, in the rest reference frame of the electron, as the result of the interaction of the spin magnetic moment
In physics, mainly quantum mechanics and particle physics, a spin magnetic moment is the magnetic moment caused by the spin of elementary particles. For example, the electron is an elementary spin-1/2 fermion. Quantum electrodynamics gives the ...
of the electron
:
with the magnetic field that the electron sees, due to its orbital motion around the nucleus, whose expression in the non-relativistic limit is:
:
In these expressions is the spin angular-momentum, is the Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
The Bohr magneton, in SI units is defined as
\mu_\mat ...
, is the g-factor, is the reduced Planck constant
The Planck constant, or Planck's constant, is a fundamental physical constant of foundational importance in quantum mechanics. The constant gives the relationship between the energy of a photon and its frequency, and by the mass-energy equivale ...
, is the electron mass
The electron mass (symbol: ''m''e) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about or about , which has an energy-equivalent of ...
, is the elementary charge, is the speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant that is important in many areas of physics. The speed of light is exactly equal to ). According to the special theory of relativity, is the upper limit ...
, is the potential energy of the electron and is the angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
.
Due to spin orbit coupling, a new term will appear in the Hamiltonian, whose expression is
:.
Due to this effect, electrons will be scattered with different probabilities at different angles. Since the spin-orbit coupling is enhanced when the involved nuclei possess a high atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
''Z'', the target is usually made of heavy metals, such as mercury, gold and thorium.
Asymmetry
If we place twodetectors
A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.
In the broadest definition, a sensor is a device, module, machine, or subsystem that detects events or changes in its environment and sends ...
at the same angle from the target, one on the right and one on the left, they will generally measure a different number of electrons and . Consequently it is possible to define the asymmetry , as
:.
 The Sherman function is a measure of the probability of a spin-up electron to be scattered, at a specific angle , to the right or to the left of the target, due to spin-orbit coupling. It can assume values ranging from -1 (spin-up electron is scattered with 100% probability to the left of the target) to +1 (spin-up electron is scattered with 100% probability to the right of the target). The value of the Sherman function depends on the energy of the incoming electron, evaluated via the parameter . When , spin-up electrons will be scattered with the same probability to the right and to the left of the target.
Then it is possible to write
:
:
Plugging these formulas inside the definition of asymmetry, it is possible to obtain a simple expression for the evaluation of the asymmetry at a specific angle , ''i.e.'':
:.
Theoretical calculations are available for different atomic targets and for a specific target, as a function of the angle.
The Sherman function is a measure of the probability of a spin-up electron to be scattered, at a specific angle , to the right or to the left of the target, due to spin-orbit coupling. It can assume values ranging from -1 (spin-up electron is scattered with 100% probability to the left of the target) to +1 (spin-up electron is scattered with 100% probability to the right of the target). The value of the Sherman function depends on the energy of the incoming electron, evaluated via the parameter . When , spin-up electrons will be scattered with the same probability to the right and to the left of the target.
Then it is possible to write
:
:
Plugging these formulas inside the definition of asymmetry, it is possible to obtain a simple expression for the evaluation of the asymmetry at a specific angle , ''i.e.'':
:.
Theoretical calculations are available for different atomic targets and for a specific target, as a function of the angle.
Application
To measure the polarization of an electron beam, a Mott detector is required. In order to maximize the spin-orbit coupling, it is necessary that the electrons arrive near to the nuclei of the target. To achieve this condition, a system ofelectron optics
Electron optics is a mathematical framework for the calculation of electron trajectories along electromagnetic fields. The term ''optics'' is used because magnetic and electrostatic lenses act upon a charged particle beam similarly to optical le ...
is usually present, in order to accelerate the beam up to keV or to MeV energies. Since standard electron detectors count electrons being insensitive to their spin, after the scattering with the target any information about the original polarization of the beam is lost. Nevertheless, by measuring the difference in the counts of the two detectors, the asymmetry can be evaluated and, if the Sherman function is known from previous calibration, the polarization can be calculated by inverting the last formula.
In order to characterize completely the in-plane polarization, setups are available, with four channeltrons, two devoted to the left-right measure and two devoted to the up-right measure.
Example
In the panel it is shown an example of the working principle of a Mott detector, supposing a value for . If an electron beam with a 3:1 ratio of spin-up over spin-down electrons collide with the target, it will be splitted with a ratio 5:3, according to previous equation, with an asymmetry of 25%.See also
*Spin–orbit interaction
In quantum physics, the spin–orbit interaction (also called spin–orbit effect or spin–orbit coupling) is a relativistic interaction of a particle's spin with its motion inside a potential. A key example of this phenomenon is the spin–or ...
* Mott scattering
Mott scattering in physics, also referred to as spin-coupling inelastic Coulomb scattering, is the separation of the two spin states of an electron beam by scattering the beam off the Coulomb field of heavy atoms. It is named after Nevill Francis M ...
* Photoemission spectroscopy
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in th ...
References
{{DEFAULTSORT:Sherman function Electron beam Foundational quantum physics Scattering